This is the readme.html for the model associated with the paper
Sung RJ, Wu JS, Chang HD, Wu SN, Luo CH.
Electrophysiological mechanisms of arrhythmogenic propensity in
patients with Andersen-Tawil syndrome: a simulation study.
Am J Physiol Heart Circ Physiol 2006 [Epub ahead of print].
Abstract:
Patients with Andersen-Tawil syndrome (ATS) mostly have mutations on
the KCNJ2 gene producing loss of function or dominant-negative
suppression of the inward rectifier K(+) channel Kir2.1. However,
clinical manifestations of ATS including dysmorphic features, periodic
paralysis (hypo-, hyper-, or normokalemic), long QT, and ventricular
arrhythmias (VA) are considerably variable. Using a modified dynamic
Luo-Rudy simulation model of cardiac ventricular myocyte, we elucidate
the mechanisms of VA in ATS. We adopted a kinetic model of KCNJ2 in
which channel block by Mg(+2) and spermine was incorporated. In this
study, we attempt to examine the effects of KCNJ2 mutations on the
ventricular action potential (AP), single-channel Markovian models
were reformulated and incorporated into the dynamic Luo-Rudy model for
rapidly and slowly delayed rectifying K(+) currents and KCNJ2
channel. During pacing at 1.0 Hz with [K(+)]o at 5.4 mM, a stepwise
10% reduction of Kir2.1 channel conductance progressively prolonged
the terminal repolarization phase of AP along with gradual
depolarization of the resting membrane potential (RMP). At 90%
reduction, early after- depolarizations (EADs) became inducible and
RMP was depolarized to -55.0 mV (control: -90.1 mV) followed by
emergence of spontaneous action potentials (SAP). Both EADs and SAP
were facilitated by a decrease in [K(+)]o and suppressed by increase
in [K(+)]o. beta-adrenergic stimulation enhanced delayed
after-depolarizations (DADs) and could also facilitate EADs as well as
SAP in the setting of low [K(+)]o and reduced Kir2.1 channel
conductance. In conclusion, the spectrum of VA in ATS includes (1)
triggered activity mediated by EADs and/or DADs, and (2) abnormal
automaticity manifested as SAP. These VA can be aggravated by a
decrease in [K(+)]o and beta-adrenergic stimulation, and may
potentially induce torsades de pointes and cause sudden death. In
patients with ATS, the hypokalemic form of periodic paralysis should
have the highest propensity to VA especially during physical
activities.
As extracellular K concentration was changed, the simulation
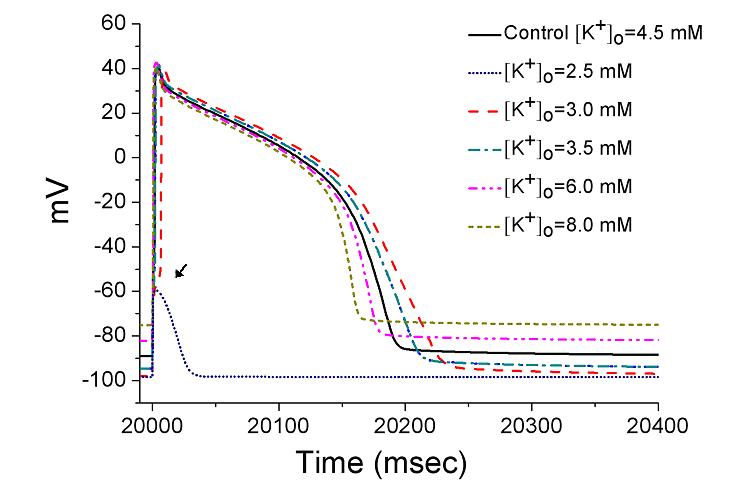 will make graphs similar to figure 7 in the paper of Sung et al.
This C++ code was subbmitted by:
Dr. Sheng-Nan Wu and Mr. Han-Dong Chang
National Cheng Kung University Medical Center
Tainan, 70101, Taiwan
snwu@mail.ncku.edu.tw
How to use:
This code can be compiled and run under linux with commands like
g++ -lm ATS-AP.cpp -o ATS-AP
./ATS-AP
After a minute or so it generates the 21 column matrix ap that
contains time(ms), voltage(mV), etc... (see program).
will make graphs similar to figure 7 in the paper of Sung et al.
This C++ code was subbmitted by:
Dr. Sheng-Nan Wu and Mr. Han-Dong Chang
National Cheng Kung University Medical Center
Tainan, 70101, Taiwan
snwu@mail.ncku.edu.tw
How to use:
This code can be compiled and run under linux with commands like
g++ -lm ATS-AP.cpp -o ATS-AP
./ATS-AP
After a minute or so it generates the 21 column matrix ap that
contains time(ms), voltage(mV), etc... (see program).
 will make graphs similar to figure 7 in the paper of Sung et al.
This C++ code was subbmitted by:
Dr. Sheng-Nan Wu and Mr. Han-Dong Chang
National Cheng Kung University Medical Center
Tainan, 70101, Taiwan
snwu@mail.ncku.edu.tw
How to use:
This code can be compiled and run under linux with commands like
g++ -lm ATS-AP.cpp -o ATS-AP
./ATS-AP
After a minute or so it generates the 21 column matrix ap that
contains time(ms), voltage(mV), etc... (see program).
will make graphs similar to figure 7 in the paper of Sung et al.
This C++ code was subbmitted by:
Dr. Sheng-Nan Wu and Mr. Han-Dong Chang
National Cheng Kung University Medical Center
Tainan, 70101, Taiwan
snwu@mail.ncku.edu.tw
How to use:
This code can be compiled and run under linux with commands like
g++ -lm ATS-AP.cpp -o ATS-AP
./ATS-AP
After a minute or so it generates the 21 column matrix ap that
contains time(ms), voltage(mV), etc... (see program).